Description
10 x 3 ml GRADUATED PIPETTES
Measurements marked up in 0.5 ml increments in these graduated pipettes.
Ideal for measuring small amounts of liquid.
- Disposable plastic transfer pipettes, 3 ml graduated
- 15.5 cm in length
- Soft, pliable and easy to use
Using a pipette can help chidden develop fine motor skills.
Scientific Sue cuts the end of the bulb off and then uses the pipettes in her Bouncing Bubble Workshop!
Some uses of pipettes for your young ones:
- Dripping coloured water onto absorbent paper
- Adding vinegar to baking soda
- Transferring coloured liquids from one container to another, say a jar to a paint palette for mixing of colours – this takes lots of concentration!
- Adding colours to a bowl of shaving or bath foam.
- Watering seeds or plants.
- Colour play – start with 2 colours. how many colours can be made? then add a third colour etc.
Plastic transfer pipettes are used to transfer liquids or oils safely into any containers without contact. This makes them ideal for transferring small amounts of liquids such as water and oils for any science or art project. They are also great for decorating cakes!
Oil and Water Experiment
- Oil (baby oil is good)
- Water
- Food colouring
- Pipettes
- Cups/bowls
- Clear bowl or dish
Secrets for Success:
- 3/4 fill you bowl with oil.
- decide on the number of colours you are going to use then collect that number of cups – using one for each colour that you want to make.
- Using a separate pipette for each colour, squirt the different colours into the bowl of oil.
- What happens?
Science in a Nutshell
The water and oil do not mix. Instead, weird effects are observed!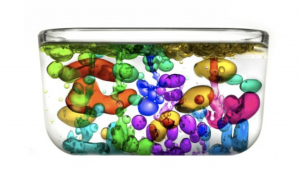
- Oil is less dense than water.
- Water is a polar liquid – meaning there is a separation of charges on the water molecules so that one end is positive and the other end is negative. These charges allow water molecules to bond together.
- Oil is a non-polar liquid.
- Salt and sugar dissolve and mix in water because they too have polar molecules. They are able to bond with the water molecules.
- However, non-polar molecules only mix with other non-polar molecules.
When we try to mix oil and water the water molecules stick toother, and the oil molecules stick together, then along with the fact that oil is less dense than water you will see that eventually the oil and water separate to form two layers. The water molecules pack closer together, so they sink to the bottom leaving the oil floating on top of the water.
When you mix your oil and water designs they will appear to stay mixed for a short time, we call this and emulsion. However, this doesn’t last long. If you look again after a minute or so, you will see the water and oil will have separated again. What colour is you water now?
Can you separate out the colours? Check out the science of chromatography!
SI Units
Scientists never just grab handfuls of chemicals and toss them together. Accurate, precise measurement is a fundamental component of good science. To standardise measurements across all scientific disciplines scientists developed the International System of Units, known as SI Units. Even with a standardised system, there is room for uncertainty in the laboratory. Minimising this uncertainty ensures proper understanding of a process or experiment.
Scientific measurements use units to quantify and describe the magnitude of something.
There are many different units for the measure of length: inches, feet, centimetres, etc.
Using common units, scientists from different countries and cultures can easily interpret each others’ results.
The current list of SI units are: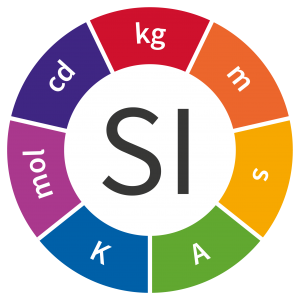
- Metres (m) for length
- Litres (L) for volume
- Kilograms (kg) for mass
- Seconds (s) for time
- Kelvin (K) for temperature
- Ampere (A) for electrical current
- Mole (mol) for amount of substance, and
- Candela (cd) for luminous intensity.















